
Unit 3 Objectives:
Topic: Soil types and Soil Structure
Lecture Objectives:
Introduction:
In the geotechnical field it is essential to gain a knowledge of the nature of
soil and its components. To understand even the most basic concepts of soil
structure and soil deposits it is advantageous to understand some of the most
fundamental principles of soil mechanics. In nature, soils are made up of
particles of varying size and shape. Size and to some extent, shape are factors
that have been found to be related to, or to affect, the material behavior of
soil to some degree. Throughout this unit we will look at how and why soils are
categorized the way they are along with a look at some special soil types that
are found in this area.
What is a soil?
The term soil generally refers to the random accumulation of particles of disintegrated rock caused by erosion or some form of weathering. A soil can also be the result of man made mechanical crushing or through chemical processes.
How do we categorize soils?
Due to a wide variation in physical characteristics and behavior, soils have been subdivided into categories based on their physical properties such as:
For the most part, soils are categorized relative to their particle size. However, there are particles present in soils that are to small to be seen visually. These particles are categorized by their plasticity limit which we will discuss in a moment.
Other influences that affect a soils behavioral properties include:
Usually the simple typing of soil provides sufficient information for design and construction, whereas certain other conditions require that detailed information about the soils composition and structure be determined.
The major categories of soil include:
Gravel and Sand are considered to be a coarse grained soil due to the fact that their individual particles are large enough to be distinguished without magnification.
Silts and Clays however, are considered to be a fine grained soil as a result of
their small particle composition.
Silt and Clay particles for the most part can not be viewed unless they are
magnified.
The most commonly used divisions for classifying soils for engineering purposes are shown in the table below.

Particles that are larger than gravel are referred to as cobbles or boulders.
It standard practice to indicate the soil aggregate requirements on the basis of size instead of or in addition to classification.
The bottom line…..Particle size serves as the basis for the classification of
sands, gravels, cobbles, and boulders.
Classification of Fine Grained Soils:
Unlike the classification of coarse grained soils, fine grained soils are classified by a means of rating their limits of plasticity. Plasticity is a property possessed by a fine grained soil that is exhibited in the presence of water. For the engineering purposes of classifying a fine grained soil, the first question that should be asked and answered is:
Does the material have properties of plasticity or nonplasticity?
Clay soil is plastic over
a range of water content; that is the soil can be remolded or deformed without
causing cracking, breaking, or change in volume and will retain the molded
shape.
Other clay properties would include:
Silt soil on the other hand has little or no plasticity and when dried has very little strength. A silt can be identified by the number of different hand methods which we will explore in the lab. Hand methods of classifying a fine grained soil as either a silt or clay include:
Why is There a difference Between Clays and Silts?:
The primary reason for the difference in behavior between clay and silt is the result of the difference in mineralogical composition of the soil types and the particle shape. Silt soils are very small particles of disintegrated rock, as are sands and gravels and posses the same general shape and mineralogical composition as sands and gravels which also are nonplastic.
Clay minerals represent chemical changes that have resulted from the decomposition and alteration of the original rock minerals. The effect is that their size and shape are significantly different from those of other types of soil particles.
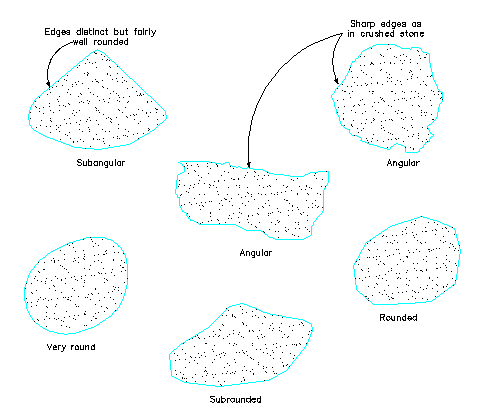
Particle Shapes and Sizes:
Particles in the sand, gravel, and boulder categories are considered a bulky
grain, indicating that particle dimensions are approximately equal; That is, all
dimensions in the length, width, and thickness directions are roughly the same.
Individual particles are frequently very irregular in shape, depending on the
type of rock they were derived from, the age, and exposure to weathering and
transporting process. Generally, a new particle is angular and rough surfaced,
and with time and weathering the particle is modified to become smooth surfaced
and rounder.
Angular shaped particles possess better engineering properties, such as higher
shear strength than do weathered and smooth particles.
The mineralogical structure of a true clay is very different from the mineral components of other soil types which results in the distinctions of clay minerals and non clay minerals.
Clay Minerals – Are typically the result of an alteration or metamorphic change of the rock minerals. Almost all clay minerals are crystalline minerals which are an orderly and repetitious arrangement of molecules to produce a sheet like structure that is capable of developing cohesion. This cohesion is the result of an attraction between the soil sheets and any water that may be present in the soil. An individual clay particle may be the biproduct of many sheet like structures stacked on top of one another.
Non-Clay Minerals – Due to a sedimentary origin of fine grained soil deposits and overlap in sizes of the clay and non clay minerals, it is unusual to find natural deposits of pure clay mineral soils. Very often, clay deposits are a mixture of clay minerals and non clay minerals. Therefore the term clay mineral has resulted to prevent confusion when referring to soil deposit that has the general properties of cohesion and plasticity.
Clay minerals vary in their composition and therefore in their behavioral
properties. The predominant types of building blocks or constituent sheets that
combine to form different types of clay minerals are:
Explanation of Isomorphic Substitution:
| Clay Mineral | Composition | Layer Thickness | Shape of Mineral, General Properties, and Comments |
| Kaolinite | One silica, one alumina sheet. Very strongly bonded together. | 7.5A | The most prevalent clay mineral. Very stable with little tendency for volume change when exposed to water. Kaolinite layers stack together to form relatively thick particles. Particles are plate shaped. Form from crystalline rocks in humid climates. |
| Halloysite | One silica, one alumina sheet make up the layer. Has a sheet of water molecules between layers. (Similar to Kaolinite except for sheet of water.) | 10A | Sheets of halloysite curl into tubes. Strength and plasticity are significantly affected by drying and removal of water. After drying, the clay mineral will not reinstate a water layer if again exposed water. |
| Illite | Alumina sheet sandwiched between two silica sheets. Potasium provides the bond between layers. | 10A | Irregular flake shape. Generally more plastic than kaolinite. Does not expand when exposed to water unless a deficiency in potassium exists. Illite clays seem most prevalent in marine deposits and soil derived in from micaceous rock. |
| Montmorillonite | Alumina sheet sandwiched between two silica sheets. Iron or magnesium may replace the alumina in the alumina sheet; aluminum may replace some silicons in the silica sheet (isomorphous substitution). Weak bond between layers. | 9.5A | Irregular plate shapes or fibrous. Because of the weak bond between layers and the negative charge resulting because of isomorphous substitution, the clay readily absorbs water between layers.Has a great tendency for large volume change because of this property. Forms mainly from ferromagnesium rock aand develops mostly in semi-arid and temperate climates; also from the decomposition of volcanic ash. |
| Chlorite | Alumina sheet sandwiched between two silica sheets, but layers are bonded together with an alumina sheet. | 14.1A | Irregular plate shapes. Nonexpanding. Formed from well-drained soils and micaceous rocks in humid areas. |
| Note: 1A = 1 x 10 ^-7 |
The Relationship Between Groundwater and Clay:
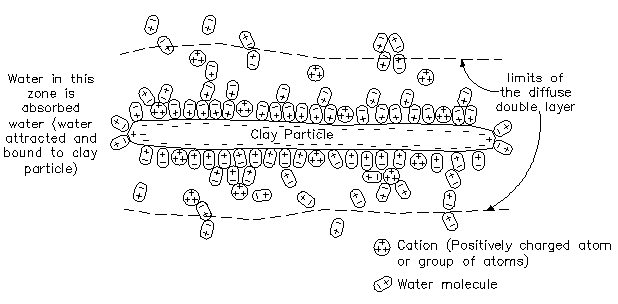
To begin with, surfaces of a clay mineral particle have a net electrical charge
that is negative, while the edges have a positive and negative charge. This is
the result of molecular grouping and arrangement of ions.
The effect of groundwater is such that it is rarely pure and very often contains dissolved gases, minerals and other compounds in solution or in suspension which result in acidity.As a result, the groundwater for an area may be acidic (pH less than 7) or alkaline (pH greater than 7) instead of neutral which would have a pH of 7. Due to the presence of moderate to higer levels acidity or alkalinity in ground water the various rocks and minerals will disassociate or break down into component cations (positively charged ions groups of atoms) and anions (negatively charged ions), which are then transported by the groundwater to react with minerals at other locations to form new minerals.
Normally, clay minerals by themselves would tend to repel each other due to the net negative charges present on the surface, unless edge to surface contact were made. Due to the net negative charge of the of the clay particle there will be an attraction of positively charged cation which are present in potassium, sodium, calcium, and aluminum contained in the soil moisture thus creating a balanced or equilibrium condition. In addition, because of the net positive charge of the cations, they in turn can also attract negative charges. As a result of this phenomenon, water becomes bonded to the cations. The negative tips of the water molecules are attracted and held to the cation, which inturn is held by the clay particle. The resulting effect is that significant water becomes bonded to the clay. Water molecules are also held to the particle surface, where they become attracted directly to a location of negative charge.
Soil structure can become a very important factor when considering a particular
soil for it’s suitability as a construction material.
When considering a material with uniform or similar sized particles in a loosely
consolidated condition we could expect to find a very high void ratio and
therefore a very low density.
In nature, actual soil deposits are made up of random accumulations of soil particles having some variation in particle size. With a wide variety or good gradation of soil particles we can expect a lower void ratio and therefore a higher density.
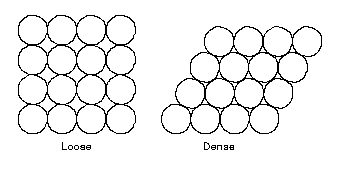
When relating the volume of void spaces to properties desirable for building construction purposes, it is generally anticipated that the lower the void ratio, the higher the strength and the lower the compressibility. With greater soil strength means higher bearing capacity and higher allowable stress factors.
Coarse soil in an initially loose condition may be prone to quick volume reductions and loss of strength if subjected to shock or vibrations, unless there is some cementing agent at points of particle contact or cohesive strength.
We know from experience that sands or silts can be deposited in such a manner that a Honeycomb structure may result. A Honeycomb structure is basically a network of loose particles with concentrated pockets of entrapped air. Grains settling slowly in quiet waters, or loosely placed moist soil, can develop a particle to particle contact that bridge themselves over relatively large void spaces between the aggregates. This would have a negative effect for overlying material. ie (Collapse)
Flaked-shaped particles such mica flakes, which are present in coarse-grained soils, are predominantly responsible for the bridging effect resulting in the large void ratios. When subject to external loading, however, the flakes are incapable of providing great support, bending, under a load.
In comparing coarse-grained soils and silts to the finer clay particles we find that the mass of an individual coarse-grained soil and silt particles is relatively great compared to the surface area. As a result, the effect of gravity has the most influence over the arrangements of soil deposits. However, Clay particles have a large surface to mass ratio and more effected by the electrical forces acting on their surfaces.
The term flocculation refers to the assembling of coagulated particles into floc particles. Flocculation may be partly a chemical bridging mechanism, enhanced by the use of substances like poly-electrolytes.
Explanation of Flocculation:
Flocculation
Flocculent Structure:
Clay deposits developed from clay particles that have settled out of suspension in a freshwater or saltwater environment develop an attraction or contact between many clay particles through an edge to face arrangement. Clays that settle out in a salt water solution tend to have a more flocculated structure than do particles that have settled out in a fresh water. The more flocculent structure in the saltwater solution is due to the presence of the salinity which acts as an electrolyte. The electrolytes reduce the natural repulsive forces of the clay particles and increase the surface to edge contact bond. In considering the fresh water arrangement of clay particles, there is still a flocculent structure, however the particles are structured in a more parallel arrangement.
Clay deposits with flocculent structures will have high void ratios, low density, probably high water contents. The structure, however, is quite strong and resistant to external forces because of the attraction between particles. If there is change in environmental conditions such as the leaching out of the present salts, the attraction and strength is substantially decreased.
Clays that have been further transported after being deposited, as a result of glacial action, are reworked or remolded by the transportation process. The particle structure that develops from remolding is a more parallel arrangement of particles than existed in the floculent condition. This type of arrangement is called a dispersed or oriented structure.
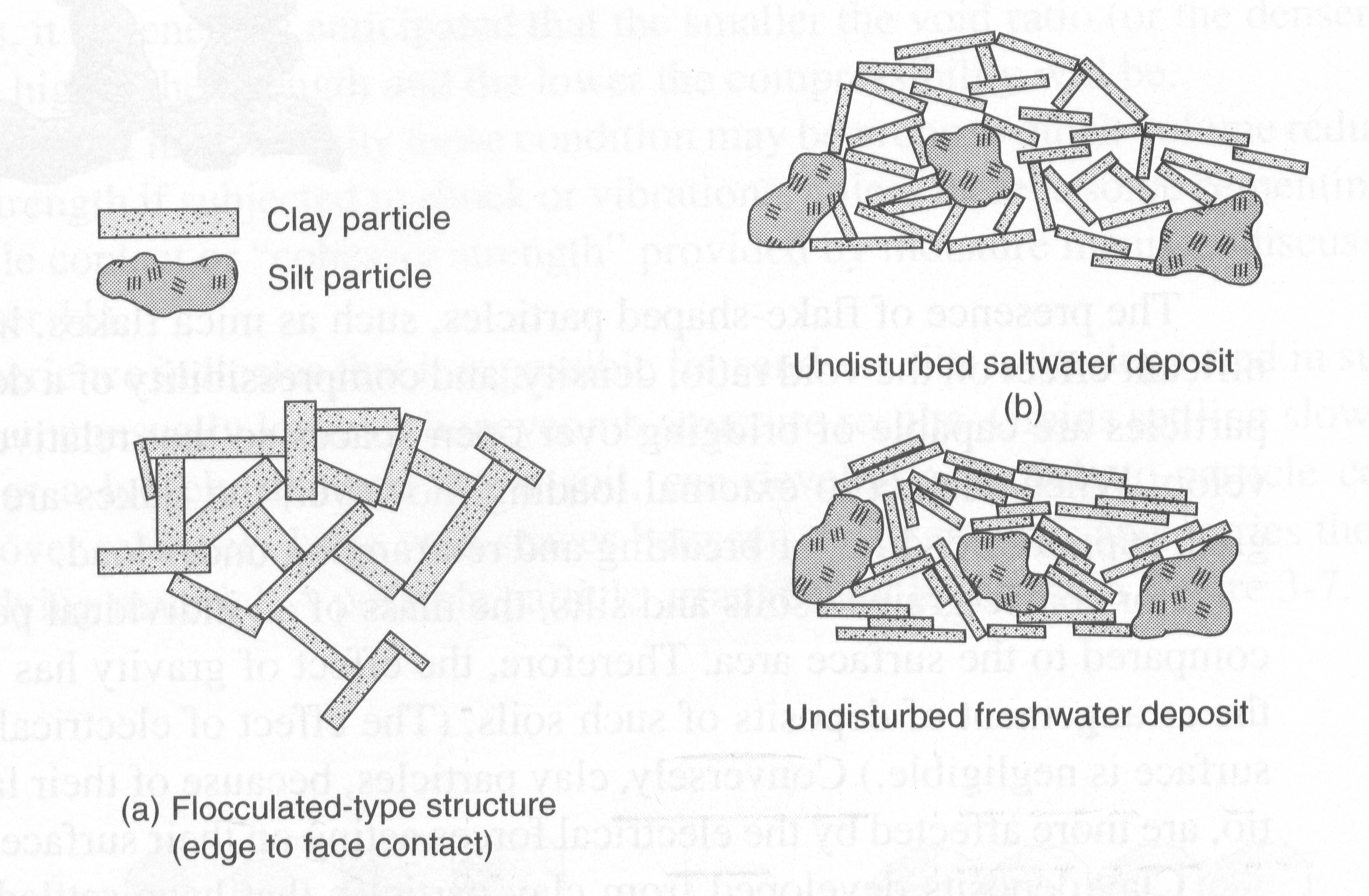
Flocculated particle structures
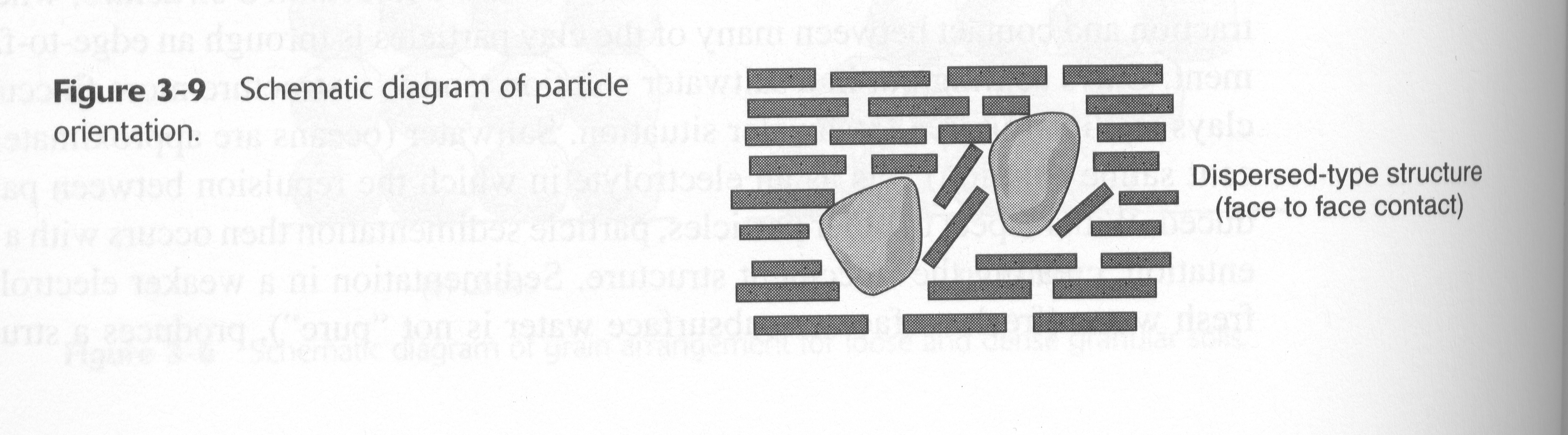
Dispersed particle structures
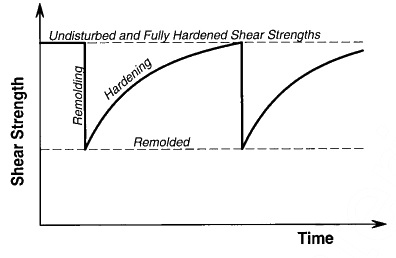
Is defined as a "process of softening caused by remolding, followed by a time-dependant return to the original or harder state". The degree of difference between the undisturbed strength and remolded strength, and the extent of strength gain after remolding, are affected by the type of clay minerals present in the soil.
For many construction situations, Thixotrophy is considered a beneficial phenomenon, since with the passing of time soil structures and disturbed foundation soils get stronger and safer with the passing of time.
Further Information on Thixotropy: Thixotropy
In the construction industry, special soils are used to describe soils that have unique properties or have unusual behavioral properties that are capable of causing problems and therefore require special treatment.
Collapsible Soils:
A collapsible soil refers to the category of soil deposits that experience
significant decrease in volume when exposed to water. These types
of soils are predominantly found in arid regions around the world.
A property of the collapsible the collapsible soil is the presence of silt or
clay fine grained materials which act as a binder to produce a fragile structure
vulnerable to breakdown in the presence of water.
Liquefaction:
Liquefaction is a condition that can occur when saturated cohesionless sand deposits exist in a relatively loose condition. If subjected to vibration or shock waves the soil grains move quickly to into a more dense and compact arrangement, but the presence of void space water interferes and prevents the particle to particle contact.
| Video Showing Liquefaction: |
|
|
Expansive Clays:
The generic name for clay minerals that swell or shrink as changes soil water content occur is called smectite. Montmorillonite is one of the prominent smectite clay minerals found worldwide.
Clays containing the montmorillonite mineral expand in volume if the soil water
content is below the stability value. On the other hand, if there is a deficit
of water below the stability level, there will be considerable shrinkage of the
material.
These expansive and shrinkage properties can have a considerable affect on
structures and result in serious damage.
Chemical stabilization can be achieved by using additives the reduce the soils
properties to attract or loose moisture. A popular additive used for this
purpose is lime.
Dispersive Clays:
Fine-grained soils that will defloculate if exposed to still water and erode if
exposed to low velocity water are called dispersive clays. Usually clays do not
erode unless the flow velocity is relatively high.
A clays susceptibility to dispersion has been found to be related to the
presence cations in soil pore water. Generally, the repulsive forces between
clay particles that act to cause deflocculation decrease as the concentration of
ions increases.
The erodibility of dispersive clays can be reduced through the use of hydrated
lime or aluminum sulfate to treat the soil.
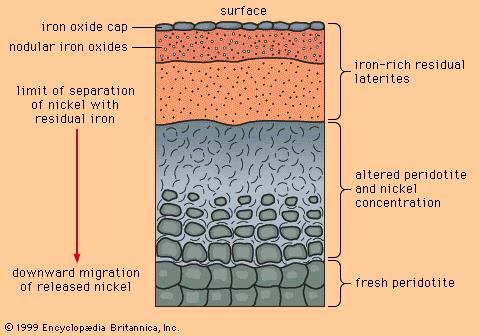
Laterites:
Refers to a category of residual soil formed from the weathering of igneous rock under conditions of high temperature and high rainfall. These soils are predominantly found in the tropical regions, where the decomposition process results in a soil leached of silica and calcium carbonate but retaining high concentrations of iron and aluminum .
 |
 |